Stem cells and liver regeneration
The liver has the possibility to regenerate after pharmacological or surgical damage. This fact has been known since Greek mythology and makes the liver a very special organ. This ability is used in surgery today by various stimulating methods, e.g., portal vein embolization or ALPPS in the context of major liver resections. However, the regenerative capacity differs between patients, sometimes considerably in case of fatty liver or cirrhosis. Reduced regenerative capacities can be associated with a considerably high risk of liver insufficiency for the individual. In this respect, a better understanding of the underlying mechanisms is urgently needed to push the possibilities of extended liver surgery even further, and to make liver directed therapies safer for the patients.
Stem cells in liver regeneration
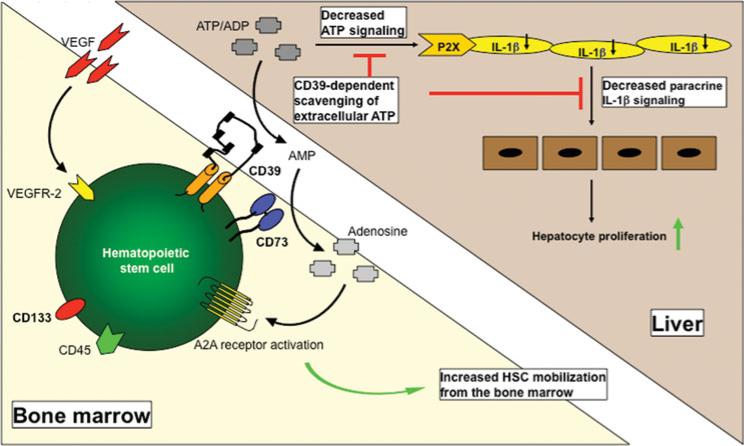
Our research group has been studying mechanisms of liver regeneration for 15 years, focusing on the possible role of stem cells. This makes us one of the first to have dealt scientifically with stem cell-mediated liver regeneration. We have learned that stem cells can be detected at high levels in the blood after liver damage, being indicative for a potential physiological significance in the context of liver regeneration. Over the next years, were able to describe several cellular subsets involved in the context of liver regeneration. In particular, CD39 positive cells seem to play an important immunomodulatory role by scavenging extracellular ATP (Cartoon 1). We also administered stem cells in mouse models and a preliminary series of patients and could confirm regenerative effects for both mesenchymal and hematopoietic stem cells.
Stem cells shed microparticles
Mobilization of stem cells after liver injury takes a few days, which might be too long after severe liver injury. Already a few hours after liver damage, however, small cellular components of stem cells can be detected in the peripheral blood. This is interesting in that these microparticles resemble the cells of origin both in terms of their surface profile and their cellular content. We were able to prove that cells from the bone marrow can in principle modulate other cells, such as endothelial cells, by fusing with them through the release of such microparticles (Cartoon 2). This transfer of distinct cellular information (stem cell-derived mRNA, miRNA, proteins…) through microparticles is extremely important in terms of a better understanding of intercellular communication in liver regeneration and also as a possible therapeutic alternative to cellular therapy.
Ongoing efforts
We are convinced that stem and immune cell therapy can make a hopeful contribution to improving regeneration after injury and increasing the safety of liver resections in humans. We have been able to put together important pieces over the last few years, supported by the generous funding by the Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung and American Liver Fundation. However, a better understanding is urgently needed to bring cellular therapies into the clinic. We are currently choosing translational approaches to confirm results from basic science studies in patients. The primary goal is to identify a therapy that is as efficient, cost-effective and safe as possible. Our efforts are directed towards testing cellular therapy to support liver regeneration in clinical trials in the near future.
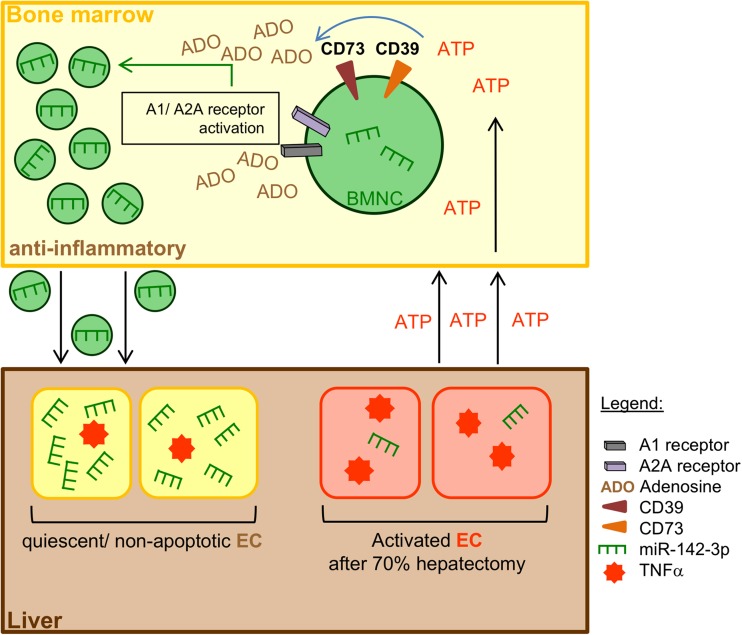
Proposed mechanism of intercellular communication between BM-MNC and endothelial cells (EC) during liver regeneration. Release of the immunomodulatory miR-142-39 into BM-MNC-derived MP is modulated by the degradation of extracellular ATP to adenosine. This miRNA encapsulation can be boosted via A1 and A2A receptors. Transfer of miR-142-3p via the fusion of enriched MP with activated/apoptotic EC diminishes TNF-α mRNA levels and limits endothelial apoptosis. Inactivation of EC may also lead to a kind of feedback mechanism to prevent an abundant immune response. BM-MNC, bone-marrow-derived mononuclear cells; EC, endothelial cells (Kuhn et al. Purinergic Signal. 2018)




