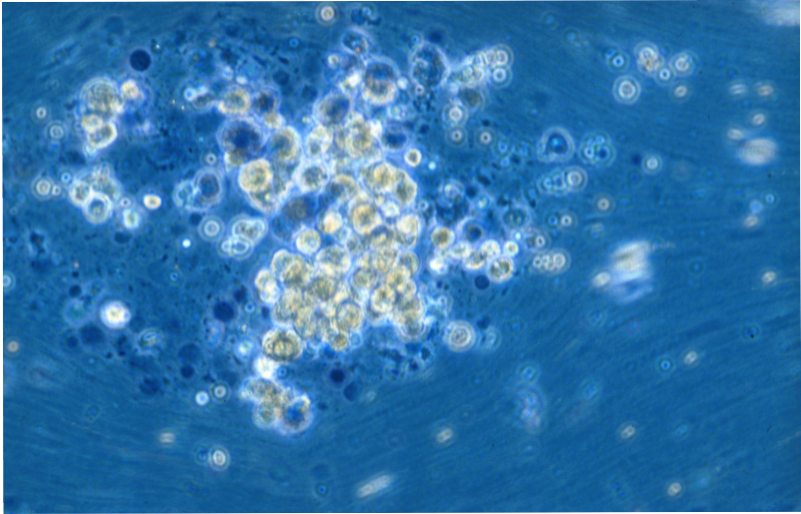
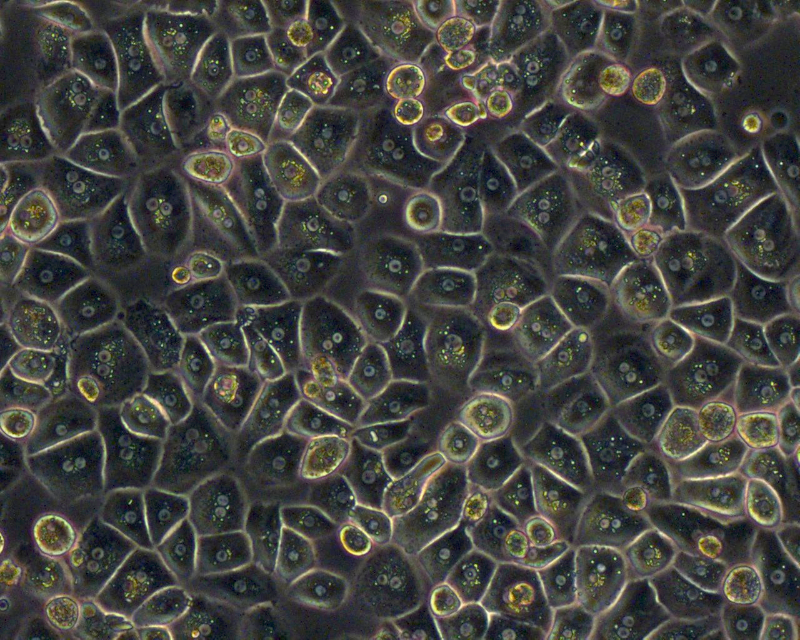
Liver Cell Isolation & Culture
Liver cells from human or animal origin are required for basic and translational research. In vitro studies enable to study the effects of pharmaceuticals or chemicals on liver cells. Physiological principals and mechanisms of liver regeneration and development can be investigated in detail. Moreover, in vitro studies with liver cells contribute to the efforts to reduce the number of animal experiments. As well, primary liver cells are required for clinical applications such as bioartificial liver support, liver cell transplantation or in vivo tissue engineering. Since diseased livers are more and more used for organ transplantation, isolation of human hepatocytes is increasingly limited to surgical specimens or marginal livers. Thus, hepatocyte isolation, culture and cryopreservation needs to be further evaluated and optimized.
Our lab has a broad experience in the field of liver cell isolation and culture. Liver cells are isolated from human, porcine and rat livers and studies are performed in conventional two-dimensional culture systems as well as more sophisticated bioreactor systems.
Our lab has a broad experience in the field of liver cell isolation and culture. Liver cells are isolated from human, porcine and rat livers and studies are performed in conventional two-dimensional culture systems as well as more sophisticated bioreactor systems.
Primary Human Liver Cells
Isolation and culture of primary human hepatocytes
Primary human hepatocytes are isolated from human liver specimen obtained from patients undergoing partial hepatectomy with informed consent of the tissue donor and following the ethical and institutional guidelines. A modified two-step collagenase perfusion technique is used for hepatocyte isolation. Hepatocytes are purified using percoll density centrifugation. Human hepatocytes are cultivated on cell culture plastic ware, in temporary suspension culture using the Rotary Cell Culture System (RCCS), or in the SlideReactor.
Criteria for identification of the most promising liver specimen for human hepatocyte isolation
In a study with specimen from 50 patients, the influence of donor liver characteristics on the isolation outcome and cell function of freshly isolated hepatocytes was investigated. The isolation protocol used resulted in a mean cell yield of 18.7 ±1.7 x106 viable hepatocytes/g liver. The average viability after isolation was 82.5 ± 1.3%. Donor age significantly affected the isolation outcome, but was not found suitable for predicting cell yields. Preoperative blood parameters did not correlate with cell yield, although cell function was affected: total protein, albumin synthesis, and cell viability were significantly decreased for serum gamma-glutamyl-transferase (GGT) levels >60 U/L. Specimens from patients with benign diseases gave significantly higher cell yields than tissue removed due to secondary and primary tumors, respectively. The indication for surgery was identified a valuable basis for identifying the most yielding specimens. Hepatocytes from donors with high GGT levels appeared to show reduced functional properties.
For more detailed information, see Artif Organs. 2008 Mar;32(3):205-13
For more detailed information, see Artif Organs. 2008 Mar;32(3):205-13
Cryopreservation of primary human hepatocytes
Problems with the limited availability of human hepatocytes for cell transplantation may be overcome by efficient cryopreservation techniques and formation of appropriate cell banking. We investigated the effect of the disaccharide trehalose on the cryopreservation of human hepatocytes. Liver cells were frozen in culture medium containing 10% dimethyl sulfoxide (DMSO) that was supplemented with varying concentrations of trehalose. During the postthawing culture period, viability, plating efficiency, total protein, cell proliferation, enzyme leakage, albumin and urea formation, as well as phase I and II metabolism were analyzed. The use of trehalose as an additive for cryopreserving human hepatocytes resulted in a significantly increased total protein level in the attached cells, higher secretion of albumin and a lower aspartate aminotransferase (AST) level after thawing. In conclusion, the use of trehalose as cryoprotective agent significantly improved the outcome of human hepatocyte cryopreservation.
For more detailed information, see Liver Transpl. 2007 Jan;13(1):38-45.
For more detailed information, see Liver Transpl. 2007 Jan;13(1):38-45.
Primary porcine hepatocytes
Due to the increasing shortage of organs for liver transplantation, marginal livers are increasingly used for transplantation. Thus, there is a need for animal sources of hepatocytes for toxicological and mechanistical studies or studies where the human origin is not essential. Moreover, large animal models are necessary to address specific questions of LCT.
We modified a protocol for isolation of porcine liver cells from van de Kerkhove et al. The perfusion and digestion protocol was adapted to human hepatocyte isolation. Viability of porcine hepatocytes isolated using this optimized protocol was comparable to the viability of human hepatocytes. Porcine hepatocytes have been used for both in vitro experiments and preclinical studies in the swine model.
For more detailed information please refer to
Tissue Eng Part C Methods. 2009 Dec;15(4):681-6.
Cell Med. 2010 1(3):123–135
We modified a protocol for isolation of porcine liver cells from van de Kerkhove et al. The perfusion and digestion protocol was adapted to human hepatocyte isolation. Viability of porcine hepatocytes isolated using this optimized protocol was comparable to the viability of human hepatocytes. Porcine hepatocytes have been used for both in vitro experiments and preclinical studies in the swine model.
For more detailed information please refer to
Tissue Eng Part C Methods. 2009 Dec;15(4):681-6.
Cell Med. 2010 1(3):123–135
Primary Rat Liver Cells
The rat model is often used for experimental studies addressing liver regeneration or LCT because experiments are more easily performable in the rat than in the smaller mouse. We established a protocol for isolation of hepatocytes from rats. This protocol has already been successfully used for isolation of hepatoyctes from regeneration rat livers following 70% partial hepatectomy in order to investigate in detail microRNA expression changes in hepatocytes.
For more detailed information please refer to
Am J Physiol Regul Integr Comp Physiol. 2011 Jun;300(6):R1363-72.
For more detailed information please refer to
Am J Physiol Regul Integr Comp Physiol. 2011 Jun;300(6):R1363-72.