Sterile inflammation and innate immune responses in acute-on-chronic liver failure
Acute on chronic liver failure (ACLF) is defined as an acute hepatic insult in patients with chronic liver disease, often cirrhosis, and is characterized by high death rates and little in way of therapeutic options. Systemic inflammation is considered a hallmark of ACLF and can be linked to progression of liver failure and clinical deterioration. Patients without characteristics of systemic inflammatory response syndrome (SIRS) and no demonstrable sepsis, e.g. increased heart and respiratory rates and other surrogate markers of infection, show significantly better outcomes.
Modulating inflammation in ACLF
Criteria for ACLF and SIRS substantially overlap and support the assumption of mechanistic similarities between both syndromes. The inducers of inflammation are either exogenous (pathogen-associated molecular patterns; PAMPs) or endogenous (damage-associated molecular pattern; DAMPs) and their profiles of these are linked to progression of the disease. Thus, modulating inflammation and the linked immune responses in ACLF might help to restore homeostasis and improve of regenerative capacities of the injured liver.
GRAFT study
The GRAFT study (PI Cornelius Engelmann, Berlin/ Thomas Berg, Leipzig) was an investigator initiated, multicenter, randomized, controlled trial, which recruited patients with ACLF in 18 German tertiary centers between March 2016 and April 2019. Patient with ACLF aged ≥ 18 were eligible for the trial. Patients were randomized with a 1:1 ratio to receive either standard medical therapy (SMT) (SMT group) or recombinant G-CSF (G-CSF, Ratiograstim®) plus SMT (G-CSF+SMT group). The primary endpoint was defined as transplant-free survival 90 days after inclusion with death and orthotopic liver transplantation (OLT) counting as events.
Modulating innate immune responses in ACLF
Our group is committed to accompanying the GRAFT study with translational research, with experiments continuing far beyond the end of the study. Blood from study patients is assayed and shipped to our laboratory in Berlin for further analysis. The overall aim is to evaluate the clinical relevance of cellular and non-cellular immune responses in ACLF. Clinical evaluations are supplemented by experiments in the mouse model and in vitro. We focus on the importance of purinergic DAMPS and ectonucleotidases, such as CD39, in regulating innate immune responses and modulating exacerbated inflammation in the ACLF. Our study is generously supported by the German Research Foundation (DFG SCHM 2661/3-2).
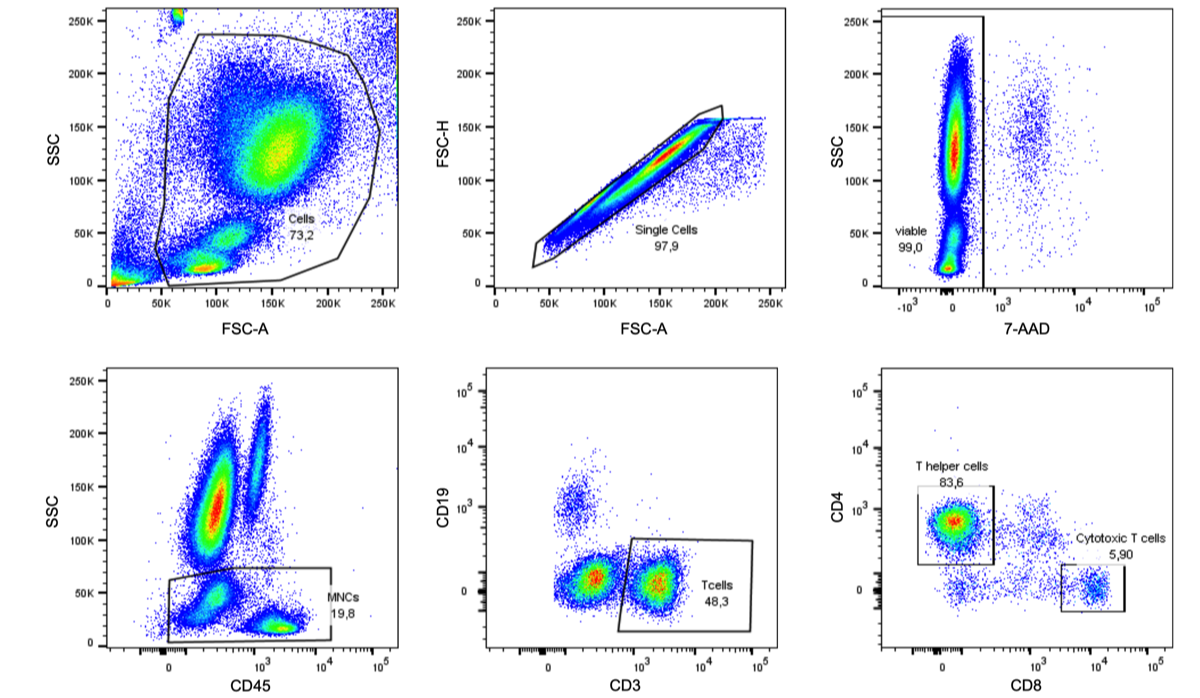
Flow cytometry analysis of blood T cells in patients with ACLF: Gate for nucleated cells based on the size (FCS-A) and granularity (SSC) of the event. To gate single cells and exclude doublets cells were further displayed in FSC-A and FSC-H. From the single cell gate viable cells were difined as 7-AAD- cells. Live mononuclear cells (MNCs) were defined as CD45+ and SSC low. CD19+ B cells were excluded from the MNCs, and the CD19-CD3+ population was defined as T cells. T cells were further divided in CD4+CD8- and CD8+CD4- and CD4 populations.
Our Team

Nadja Berndt
Student



