Signaling Pathways
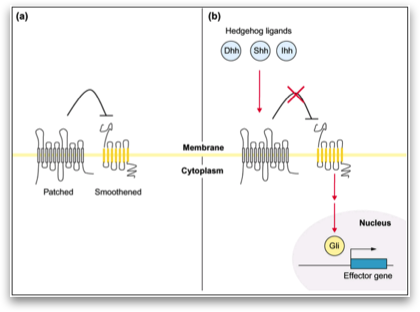
To elucidate the molecular mechanisms of pancreatic cancer, our group has steadily been working on the signal-transduction in this tumor. The objective of this project was the activation of the hedgehog signal transduction pathway, triggered by hedgehog binding to the transmembrane receptor patched 1 (PTCH1) or by mutations in the PTCH1 gene. Activation of the hedgehog signal transduction pathway, triggered by hedgehog binding to the transmembrane receptor patched 1 (PTCH1) or by mutations in the PTCH1 gene, plays an important role in the development of various tumors.
Hedgehog
Hedgehog target genes PTCH1 and GLI1 are up- regulated in pancreatic adenocarcinoma
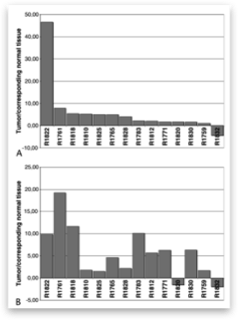
In the present study, we show that the Hedgehog target genes PTCH1 and GLI1 are up- regulated in pancreatic adenocarcinoma in comparison to re- spective normal pancreatic tissue, indicating that the Hedgehog signaling pathway is activated in this tumor entity. Hedgehog activation seems to be mediated mainly through aberrant ligand overexpression like up-regulation of SHH or IHH in pancreatic cancer cells. Interestingly, a direct comparison of SHH and IHH expression in our tumor samples with the corresponding normal pancreatic tissue did not reveal any significant differences (data not shown), indicating that alternative mechanisms might contribute to increased Hedgehog pathway activity in pancreatic cancer cells. To analyze if the Hedgehog pathway activation func- tions via alterations in the PTCH1 gene, like it is known in me- dulloblastoma21 and patients with the nevoid basal cell carcinoma syndrome,11,22 we performed the first screen for PTCH1 gene mutations in 36 pancreatic adenocarcinoma biopsies. Using SSCP- analysis and direct sequencing, we identified polymorphisms in exons 11, 12, and 22 of the PTCH1 gene, but no somatic PTCH1 mutations indicating that genetic PTCH1 alterations are not responsible for the Hedgehog activation in pancreatic adenocarcinoma.
Hedgehog inhibitors inhibit tumor growth in vitro...
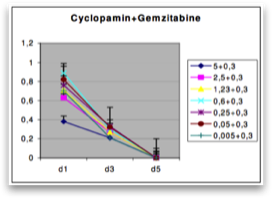
On the basis of the results of our mRNA expression analy- sis indicating Hedgehog pathway activation in pancreatic cancer, we inhibited the Hedgehog pathway with cyclopamine. To investigate whether the growth-inhibitory effect of cyclo- pamine may be related to inhibition of the Hedgehog signaling pathway, we used a GLI1 luciferase reporter assay. Transfection of the cells with a GLI1 luciferase reporter or with a nonfunctional GLI1 promoter variant indicated significant basal hedgehog ac- tivity in Capan-1 (Fig. 6A). Next, the pancreatic adenocarcinoma cells were treated with increasing amounts of cyclopamine. As shown in Figure 6B, in particular, Capan-1 showed a strong reduced viability for 3 days compared with the cells treated with PBS.
…and in vitro
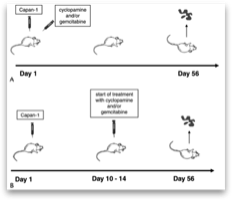
To test if the inhibition of Hedgehog signaling by cyclo- pamine identified in vitro also reduces tumor growth in vivo, we used a nu/nu nude mice xenograft model.
To determine whether growth inhibition by cyclopa- mine functions via blockade of Smo, we analyzed the PTCH1 expression in Capan-1 xenografts treated with cyclopamine. The application of cyclopamine led to a significant mRNA down- regulation of PTCH1 in the tumor tissue, indicating that cyclo- pamine treatment triggers a Hedgehog-related response in vivo. Moreover, a combined treatment with cyclopamine and the known antimetabolic agent gemcitabine resulted in a synergistic reduction of average tumor size compared with treatment of cy- clopamine or gemcitabine alone. Interestingly, previous studies indicated that growth inhibition of pancreatic cancer xenografts might be more pronounced when Hedgehog blockade was initiated concomitantly with the injection of tumor cells into nude mice. In our study, we could not confirm these findings.
Conclusion
In the present study, we show that the Hedgehog inhib- itor cyclopamine blocks Hedgehog signaling in pancreatic cancer cells in vitro and in vivo. However, it has been noted by different reports that different small-molecule inhibitors exhibit a much higher affinity for Smo than cyclopamine, they block the Shh func- tion at lower concentrations, they are water-soluble, and they have less toxic effects. Of note, small-molecule Hedgehog antagonists like IPI-269609 and GDC-0449 showed promising inhibitory effects in patients with pancreatic adenocarcinomas and other cancers like basal cell carcinomas.
We could also show modification on posttranscriptional level as Poly-(ADP-ribose)-polymerase (PARP) expression is significantly correlated with survival of patients with pancreatic cancer. In subsequent experiments we could show an increased efficiency of chemotherapy when the PARP- inhibitor 3-aminobenzamide was combined with gemcitabine.
Another promising signaling pathway is the Wnt- pathway. Similar to the Hedgehog pathway, it plays an important role both in embryogenesis and tumor development. In ongoing projects we wish to further elucidate the role of the Wnt- pathway in pancreatic cancer.
