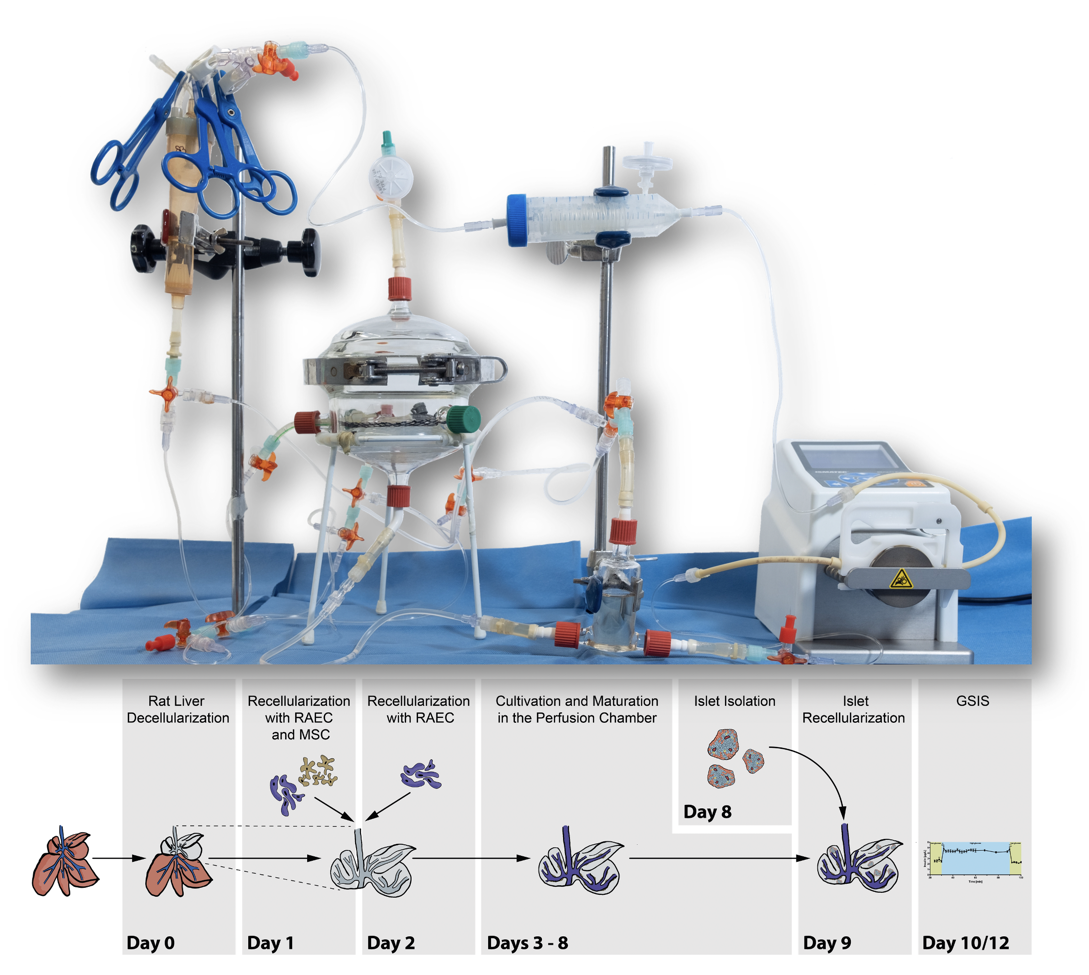
The Department of Surgery of the Charité (Director: Prof. Dr. Johann Pratschke) at the Charité Center 8 (CharitéCenter for Surgery) invites applications for the position of the Junior Professorship for Digital Surgery and interdisciplinary Technology Research (Salary Group: W1 BBesG-ÜfBE, non-tenured) with the reference number: Prof. 546/2020.
The initial appointment is for three years with the optional extension for another three years follow-ing successful evaluation. It is aimed to turn the Junior Professorship into a W2-Professorship (Salary Group: W2 BBesG-ÜfBE) after six years.The successful candidate has to fulfill the appointment requirements in accordance with § 102a of the Berlin Higher Education Act (Berliner Hochschulgesetz, Gem. § 102a BerlHG) and needs to credibly demonstrate through his/her previous scientific work that he/she is able to fulfill the expectations of the junior professorship.
One of the tasks of this Junior Professorship is the appropriate representation of the research area mentioned above. Within the framework of the
Cluster of Excellence Matters of Activity – Image Space Material, he/she is expected to evaluate, accompany and advance the digital transformation in surgery and related disciplines as well as expand the repertoire of methods and initiate innovations. In cooperation with the research areas Cutting and Material Form Function of the Cluster of Excellence, new surgical cutting techniques are to be investigated and developed. It is planned to be linked to the currently being established institutions, The
Simulated Human Being (Si-M) and the
Berlin Simulation and Training Centre (BeST). In addition to the tasks mentioned, the following three fields of activity are to be covered:
Interdisciplinary Knowledge Transfer- Implementation of new applications from areas such as deep learning, extended reality (mixed and virtual reality) or robotics in surgical practice requires an intensification of interdisciplinary cooperation
- Continuous exchange between industry and practice as well as with adjacent disciplines (e.g. Radiology)
- Integration of a growing number of applications and competencies from areas outside established medical technology, e.g.game design, computer science or human factor studies
Technology Assessment- Sustainable implementation of digital technologies through opportunity and risk assessment
- Advising the Department of Surgery on investment decisions through appropriate risk and media competency
Innovation- Identification of concrete application locations and practices of digital surgery within the clinic and experimental research (e.g. use of technologies in the context of biomedical research approaches to organ replacement as well as oncological models) for future Living Labs and to demonstrate these to the public
- Integration of users, research projects and start-ups also outside the Clinic
The successful candidate will be engaged in teaching activities of the medical education curriculum at Charité, supervise Master and Doctoral candidates, and participate in academic self-organization. In addition, the candidate should present concepts for a good supervision of doctoral students as well as for the integration of his/her research activities into the teaching of the Charité. Appointment requirements are governed by article 102a of the Berlin Higher Education Act (Berliner Hochschulgesetz:§ 102a BerlHG). Completed university degree in Natural Sciences, Humanities and/or Life Sciences or any other related field of Medicine or non-medicine is required. In addition, a Doctorate (Ph.D and/or M.D.) and significant post-doctoral experience are required. Basic medical knowledge is desired.
The Charité is an equal opportunity employer committed to excellence through diversity. As women are under-represented in academics, we explicitly encourage women to send in their application. Women will be given preference over equally qualified men (within the framework of the legal possibilities). We value diversity and therefore welcome all applications – regardless of gender, nationality, social background, religion or age. Equally qualified applicants with disabilities will be given preference.
Written applications according to the format specified on
https://career.charite.de/am/calls/application_notes.pdf should be submittedby June 19th, 2020 under https://career.charite.de. For further questions on details, please contact
Prof. Dr. Igor Maximilian Sauer.